The IVD-TEST CF reagent kit is intended for molecular genetic diagnosis of cystic fibrosis by PCR with subsequent detection of the result by capillary electrophoresis.
Cystic fibrosis is one of the most common hereditary autosomal recessive diseases among Europeans.. Mutations in the CFTR gene are the cause of cystic fibrosis. (cystic fibrosis transmembrane regulator), encoding ATP-binding protein, which forms a channel for chloride ions in cell walls. Mutations lead to impaired transport of electrolytes and chloride ions through the membranes of epithelial cells., which is accompanied by increased secretion of thick mucus and blockage of the excretory ducts of various glands.
Currently in the Russian Federation the diagnosis “cystic fibrosis” placed on one of 9 000 – 10 000 newborns. Cystic fibrosis is an autosomal recessive disease, and the disease develops only under the condition, what the child receives from the father, and from the mother a mutant version of the gene. At the same time, parents, in which one copy of the CFTR gene is intact, do not suffer from disease.
KEY FEATURES
- Registration of a test system as a medical device for in vitro diagnostics
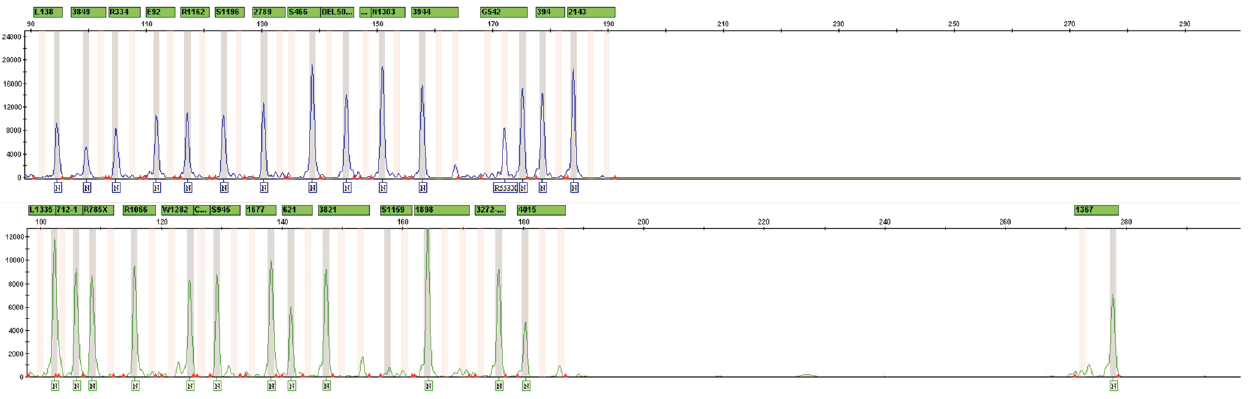
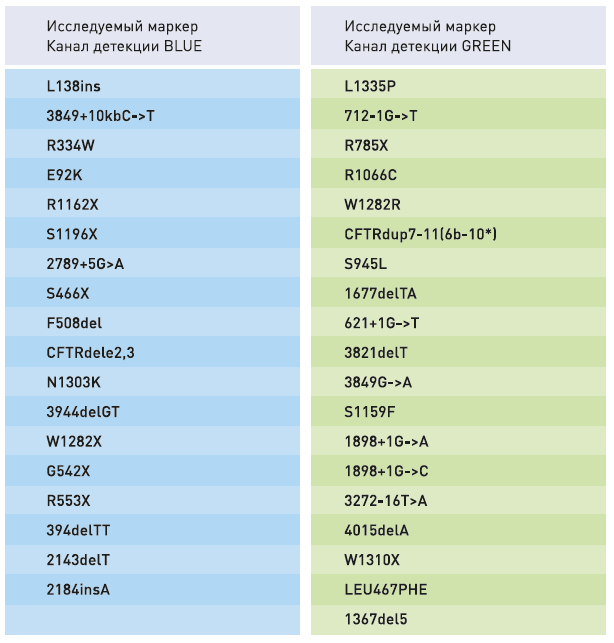
- Simultaneous multiplex analysis of 37 mutations in the CFTR gene, associated with the development of cystic fibrosis
- The panel contains known mutations in the CFTR gene with a frequency of more than 0,1%
- The possibility of identifying variants of heterozygous carriage of the studied mutations
- Analysis of the result by capillary electrophoresis
- Compatible with all modern models of genetic analyzers 3130/3500, Nanofor-05
- The kit contains all the necessary reagents for the study
- The kit was developed jointly with the Academician N.P. Medical Genetic Research Center. Bochkov"
TARGET MARKERS
REFERENCE SAMPLE