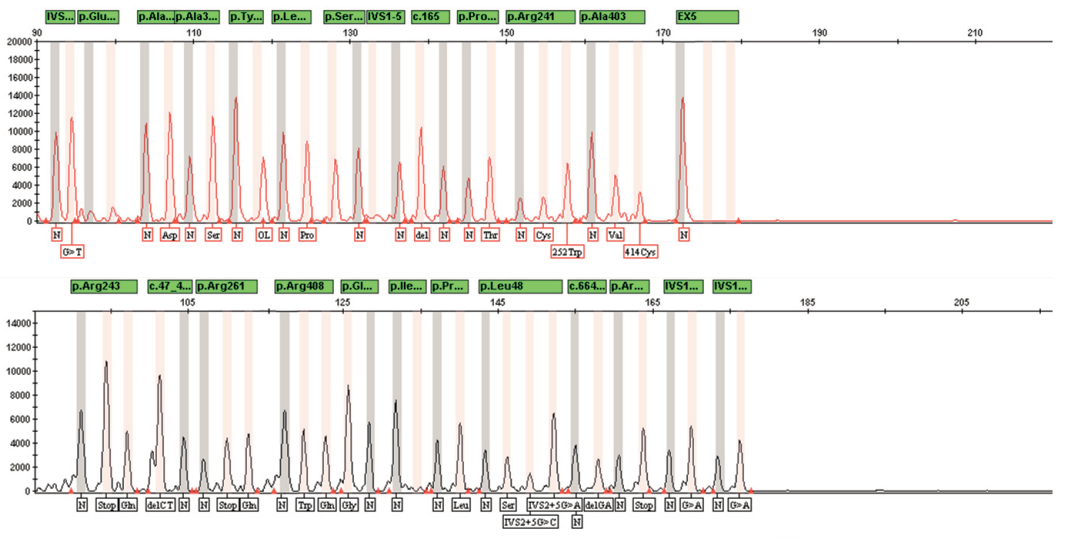
The IVD-TEST PAH reagent kit is intended for molecular genetic diagnosis of phenylketonuria and hyperphenylalaninemia by PCR with subsequent detection of the result by capillary electrophoresis.
The object of study is the region of the gene, which codes for the enzyme phenylalanine hydroxylase (PAH). Phenylketonuria is an autosomal recessive disease. Pathology is characterized by a violation of the hydroxylation of phenylalanine, accumulation of an amino acid and its metabolites in physiological fluids and tissues, followed by severe damage to the central nervous system. In most cases, the disease is associated with a sharp decrease or complete absence of the activity of the liver enzyme phenylalanine-4-hydroxylase. (encoded by the genome PAH), which normally catalyses the conversion of phenylalanine to tyrosine. In Russia, the frequency of the disease is 1 on 7 000 newborns. To 1 % cases of phenylketonuria is represented by atypical forms, associated with mutations in other genes, responsible for encoding enzymes, providing the synthesis of the cofactor of phenylalanine hydroxylase - tetrahydrobiopterin (BH4).
KEY FEATURES
- Registration of a test system as a medical device for in vitro diagnostics
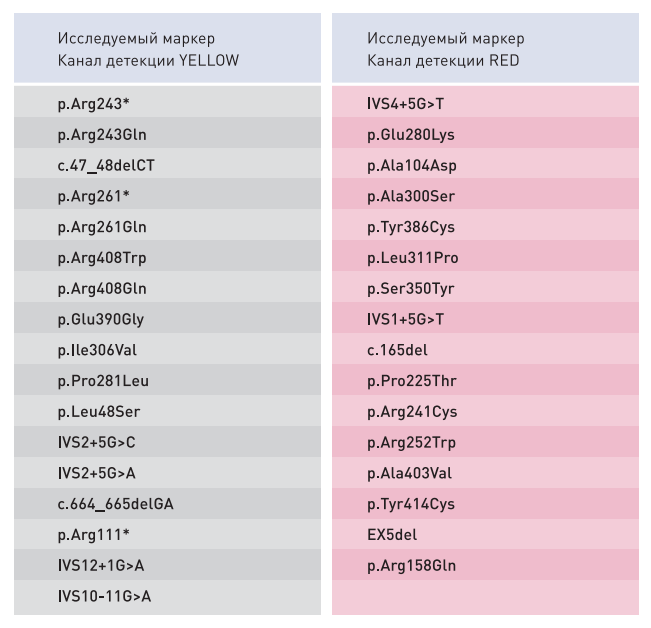
- Simultaneous multiplex analysis of 33 mutations in the PAH gene, associated with the development of Phenylketonuria
- The panel contains known mutations of the PAH gene with a frequency of more than 0,1%
- The possibility of identifying variants of heterozygous carriage of the studied mutations
- Analysis of the result by capillary electrophoresis
- Compatible with all modern models of genetic analyzers 3130/3500, Nanofor-05
- The kit contains all the necessary reagents for the study
- The kit was developed jointly with the Academician N.P. Medical Genetic Research Center. Bochkov"
TARGET MARKERS
REFERENCE SAMPLE