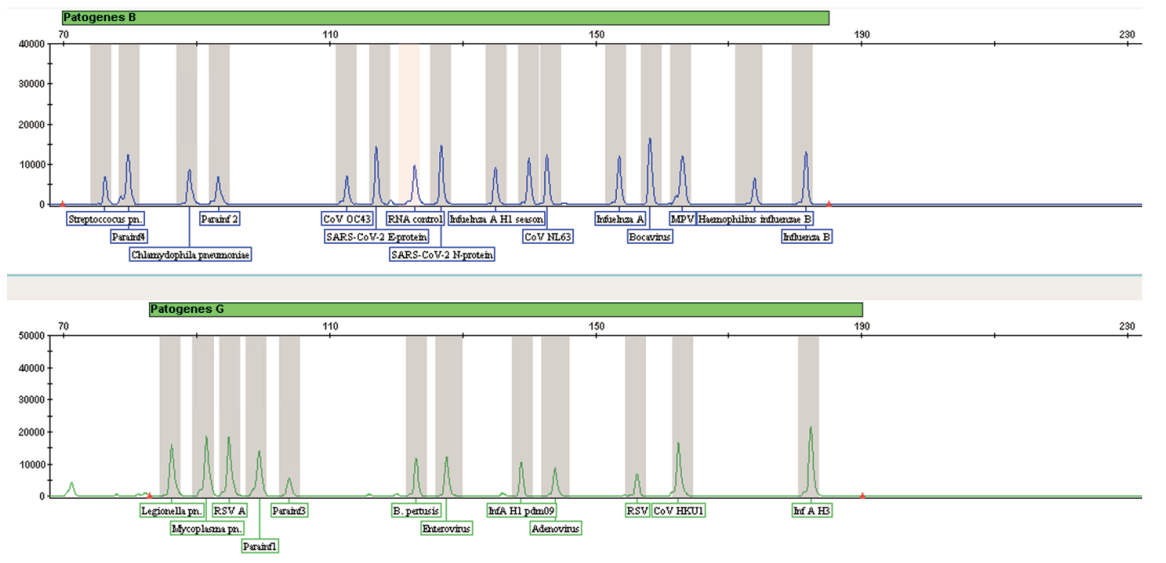
The IVD-TEST RScan reagent kit is intended for the differential diagnosis of human respiratory diseases by PCR with subsequent detection of the result by capillary electrophoresis.
Acute respiratory infections are a group of infectious diseases of the upper and lower respiratory tract., which are transmitted by airborne droplets and are characterized by symptoms of infectious toxicosis.
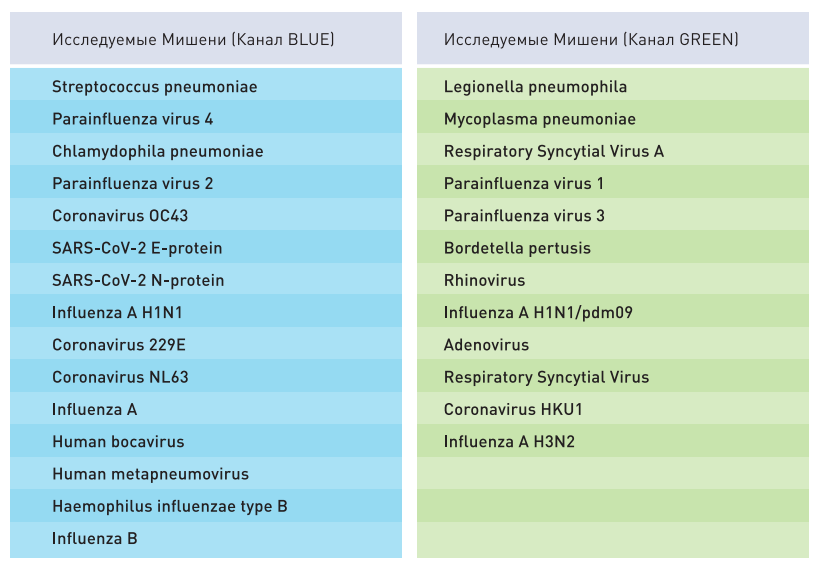
The most common pathogenic microorganisms, causing respiratory disease in humans, are parainfluenza viruses (Parainfluenza virus 1,2,3,4), coronaviruses (SARS-CoV-2, 229Well, NL63, OC43, HKU1), influenza A viruses (Influenza A H1N1, Influenza A H1N1/pdm09, Influenza A H3N2), Influenza B virus (Influenza B), rhinovirus (Rhinovirus), metapneumovirus (Human Metapneumovirus), adenovirus (Human Adenovirus), respiratory syncytial virus (Respiratory Syncytial Virus).
Given the likelihood of a patient being infected by multiple infectious agents, in order to increase the efficiency of diagnosing the disease, it is advisable to use an integrated approach to identifying the etiological agents of the infectious process using the multiplex PCR method, which will increase the information content and speed of the study.
The method of polymerase chain reaction with reverse transcription allows you to diagnose the most common diseases, caused by bacterial, and viral pathogens of human respiratory diseases. This method is based on multiplex PCR using fluorescently labeled primers, limiting conservative regions of the genomes of the studied microorganisms. The IVD-TEST RScan kit allows for simultaneous amplification 27 sections of the genomes of the studied microorganisms in the format of one multiplex reaction.
KEY FEATURES
- Registration of a test system as a medical device for in vitro diagnostics
- Detailed etiological diagnosis of acute respiratory infections
- Simultaneous multiplex analysis of 26 types and subtypes of pathogens of human respiratory diseases
- Study of a wide range of pathogens of viral and bacterial nature in one reaction
- Analysis of the result by capillary electrophoresis
- The cost of the study is comparable to existing systems for the simultaneous determination 2-3 pathogens by Real Time PCR
- Compatible with all modern models of genetic analyzers 3130/3500, Nanofor-05
- The kit contains all the necessary reagents for the study
TARGET MARKERS
REFERENCE SAMPLE